The progress of the geochemical mechanism of OA in pyrite crystals in Guangzhou
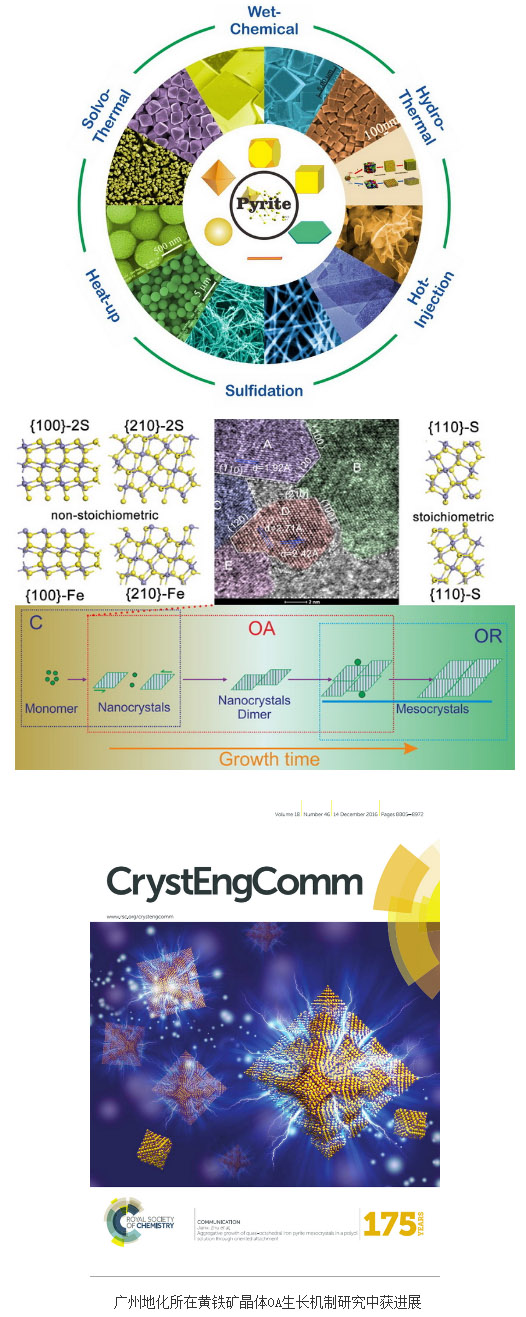
Pyrite (FeS2) is a sulphide mineral produced in the earth's crust and is widely distributed around surface and ocean hydrothermal vents. Its unique chemical composition and crystal structure determine its unique properties, wide economic value and special geochemical status. In addition to being a raw material for the extraction of traditional sulfur, sulfuric acid, and associated metal elements, pyrite is often used as a deposit-forming mineral, semiconductor photocatalytic material, environmental pollutant purifier, photovoltaic solar cell material, and lithium ion battery electrode material. Wait. In order to obtain better application performance, researchers usually use synthetic means to study their structure and application performance. The crystal growth process of pyrite in the synthesis process is the key to determine its structure and properties.
The traditional theory of crystal growth believes that the growth process of crystals grows into macroscopic crystals directly through the close proximity of the particles (including molecules, atoms, or ions). In addition to the traditional theory of crystal growth, the orientation attachment (OA) growth model developed in recent years has given entirely new contents to crystal growth methods. Mesocrystals (mesocrystals) are intermediate products that can be transformed into single crystals in the OA growth mode. They are both strong evidence for OA growth patterns and new materials with extremely high reactivity. Because mesoscopic crystals are in a metastable state, their experimental observation and characterization are relatively difficult.
Under the guidance of researcher Zhu Jianxi, PhD freshwater of the Guangzhou Institute of Geochemistry of the Chinese Academy of Sciences, a meso-type octahedral pyroclastic mesocrystal with uniform particle size was successfully synthesized in a polyol system. The mesocrystals of pyrite were characterized by high resolution transmission electron microscopy and electron diffraction. Through the tracking of its growth process, it was found that the mesoscopic crystal growth process of pyrite includes traditional crystal nucleation and growth, aggregate growth through OA mode, and Ostwald ripening process. The research results provide theoretical basis for the regulation and growth of pyrite crystals in terms of their size, morphology, and properties. Related results have been published in RSC Advances (Xian, H.; Zhu, J.; Liang, X.; He, H. RSC Advances 2016, 6, (38), 31988-31999.) and CrystEngComm (Xian, H. Zhu, J.; Tang, H.; Liang, X.; He, H.; Xi, Y. CrystEngComm 2016, 18, (46), 8823-8828), the latter was selected as the cover article. The study was supported by the National Natural Science Foundation of China (NO. 41573112) and the Innovation International Team Project (Min. Structure and Surface Physical Chemistry) of the State Bureau of Foreign Affairs/Chinese Academy of Sciences (NO. 20140491534).
Sodium street lights are known for their high efficiency and long lifespan, making them a popular choice for municipalities and other organizations looking to reduce their energy consumption and maintenance costs. They are also highly resistant to weather and other environmental factors, ensuring that they remain functional and reliable even in harsh conditions.
In addition to their practical benefits, sodium street lights also offer a number of aesthetic advantages. Their warm, yellowish light is often preferred over the harsh, bluish light produced by other types of outdoor lighting, and can help to create a more inviting and pleasant atmosphere in public spaces.
Sodium street light housing,Sodium Lamp,Metal Halide Lamp,Hid Lamp
Yangzhou M.T. New Energy & Lighting Group Co., Ltd. , https://www.mtstreetlight.com